Introduction
Community-acquired pneumonia (CAP) remains a leading cause of death from infectious diseases worldwide and a significant contributor to hospitalizations. In the United States alone, an estimated 1.5 million adults are hospitalized with CAP annually, with the elderly population experiencing the highest rates of hospitalization.1,2 Historically, CAP has been classified as either “typical” or “atypical” based on clinical presentation and etiological agents. Early in the 20th century, “typical” pneumonia referred to lobar pneumonia, primarily caused by Streptococcus pneumoniae, while “atypical” pneumonia described cases with irregular clinical courses and without the classic lobar consolidation, often caused by unknown microorganisms.3 “Atypical bacteria” refers to organisms that exhibit intrinsic resistance to beta-lactams and are unable to be visualized on Gram stain or cultured using conventional methods. A further distinguishing characteristic of these bacteria is their intracellular nature. Examples include Legionella spp, Mycoplasma pneumoniae, Chlamydia pneumoniae, Chlamydia psittaci, and Coxiella burnetii. Over time, the term “atypical” has evolved to encompass specific etiological agents rather than clinical presentation. However, there remains no global consensus on the precise definition or inclusion criteria for atypical pneumonia. The 2007 Infectious Diseases Society of America/American Thoracic Society (IDSA/ATS) Consensus Guidelines on the Management of Community-Acquired Pneumonia in Adults include viruses in this category,4 while the 2001 Japanese guidelines for the management of CAP describe atypical pneumonia with a broader range of microorganisms than the classic definition.5 In general, CAP etiology can be categorized into typical pathogens, including Streptococcus pneumoniae, Haemophilus influenzae, Staphylococcus aureus, Moraxella catarrhalis, Group A streptococci, and others; and atypical pathogens, including bacteria like Legionella pneumophila, Mycoplasma pneumoniae, and Chlamydia pneumoniae, as well as respiratory viruses.6–9
Despite advancements in diagnostic techniques, the etiology of CAP remains unknown in approximately 30% to 60% of cases.7–9 While Streptococcus pneumoniae continues to be the most common cause of CAP, Mycoplasma pneumoniae (MP) has gained increasing attention in recent years. Detection rates of MP in CAP patients range from 1% to 6%, with a notable increase in hospitalizations attributed to this organism following the COVID-19 pandemic.10 MP is an atypical, cell wall-deficient bacterium that typically causes a mild form of CAP, often referred to as “walking pneumonia”.11,12 However, there have been documented cases of severe MP-CAP leading to life-threatening complications. While MP-CAP affects all age groups, the majority of hospitalized adults in developed countries are aged 65 years or older.7 Despite its significance, a knowledge gap persists regarding the specific risk factors that predispose individuals to severe MP-CAP. In this report, we will discuss potential risk factors that may have contributed to the severity of illness in the patients presented.
Case Presentations
Case 1
A 32-year-old male from Ohio presented to our hospital with a one-week history of worsening cough and shortness of breath. His past medical history was significant for aortic coarctation status post balloon aortic valvuloplasty in May 2019, paroxysmal atrial fibrillation (PAF), aortic valve stenosis, bipolar disorder, and a history of methamphetamine and heroin use (drug-free for 5 years prior to admission). He reported ongoing tobacco, vaping, and marijuana use. He had been scheduled for an aortic valve replacement at the local Children’s hospital. The patient reported experiencing diarrhea, weakness, difficulty sleeping, and occasional sweating without documented fever. He had initially presented to an urgent care facility two days earlier and was diagnosed with a “viral illness”. Testing for RSV, influenza, and COVID-19 was negative, and he was discharged home.
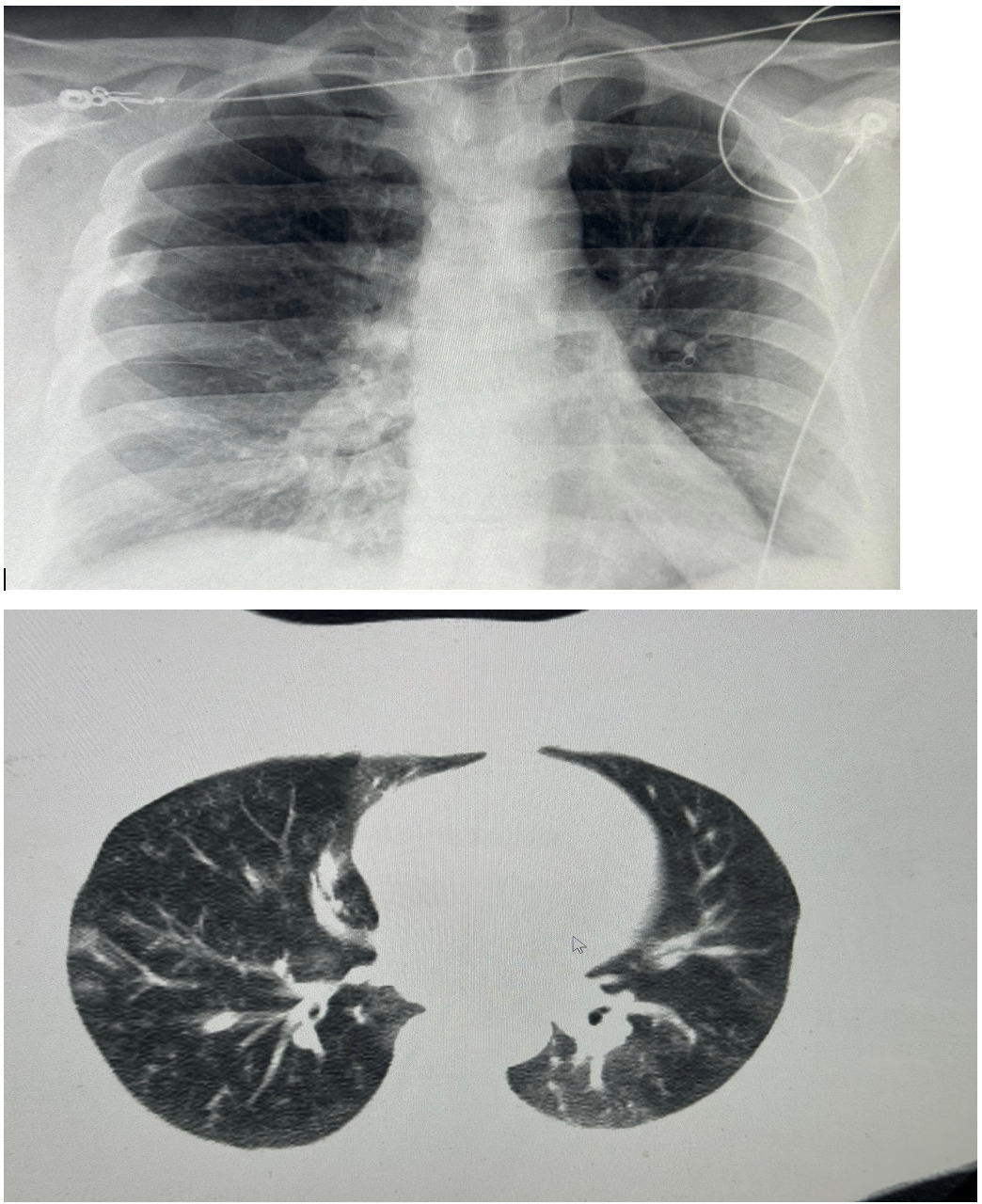
On presentation to our hospital, physical examination revealed bilateral wheezing and crackles on lung auscultation. The remainder of the examination was unremarkable. His vital signs were: blood pressure 121/70 mmHg, temperature 98.2°F, heart rate 78 beats per minute, respiratory rate 21 breaths per minute, and oxygen saturation 87% on room air which improved to >92% on 2 liters per minute of supplemental oxygen via nasal cannula. Laboratory findings were significant for mild leukocytosis (white blood cell count 12.2 x 10^9/L), with a high absolute neutrophil count (9.5 x 10^9/L) and an absolute monocyte count (1.3 x 10^9/L). His C-reactive protein (CRP) level was elevated at 264 mg/L, while his procalcitonin level was 0.08 ng/mL. HIV testing was negative. His calculated CURB-65 score was 0. Initial chest X-rays were unremarkable; However, subsequent chest CT imaging revealed a pattern characterized by small, branching nodular opacities radiating from a central line, consistent with bilateral ‘tree-in-bud’ nodular infiltrates, as shown in Figure 1. Due to persistent hypoxemia, the patient was admitted and started on intravenous ceftriaxone and azithromycin, in accordance with IDSA/ATS guidelines for CAP. A multiplex PCR on a nasopharyngeal swab was negative. However, a sputum sample yielded a positive PCR result for Mycoplasma pneumoniae on a pneumonia panel. Bacterial cultures were negative. Legionella urinary antigen and multiple fungal studies were also negative. The patient was subsequently discharged on a course of oral tetracyclines for MP-CAP and scheduled for an aortic valve replacement. The patient clinically improved.
Case 2
A 32-year-old female school teacher with a past medical history significant for morbid obesity (BMI 47) presented to our hospital with complaints of cough, shortness of breath, and fever for 5-6 days. She had initially sought care at an urgent care facility where she was prescribed amoxicillin-clavulanate and initially experienced some improvement. However, her condition worsened, prompting her to present to the emergency department. On arrival at the ED, she was tachycardic (heart rate 118 beats per minute) with an oxygen saturation of 88% on room air, requiring supplemental oxygen. Her other vital signs were stable (temperature 98.3°F, respiratory rate 12 breaths per minute). Physical examination revealed bilateral rhonchi and decreased breath sounds mainly in both bases. Had mild leukocytosis on admission with a white blood cell count of 13 x 10^9/L, and a negative serum procalcitonin of 0,03 ng/ml. The chest X-ray was reviewed and reported as normal, showing no evidence of acute pathology or abnormal findings. She was admitted and initially started on intravenous ceftriaxone and azithromycin, in accordance with IDSA/ATS CAP guidelines. A nasopharyngeal respiratory panel was positive for Mycoplasma pneumoniae. IgM titers for MP were elevated, while IgG titers were negative. A subsequent pneumonia panel was also positive for M. pneumoniae, with no other co-pathogens identified. A CT of the chest showed bilateral infiltrates, as illustrated in figure 2. She was discharged in stable condition, without the need for home oxygen, after being switched to oral doxycycline to complete a 10-day course with significant clinical improvement.
Discussion
MP-CAP is a common respiratory manifestation of infection with Mycoplasma pneumoniae.13 While MP is estimated to infect about 1% of the United States population, only 5-10% of those infected will develop pneumonia.14 The exact incidence of severe MP-CAP and the interplay of risk factors leading to severe disease remain poorly understood.
In 1976, four cases of severe MP-CAP were documented over a four-year period, all involving previously healthy women.15 A 1999 case report by Takiguchi et al.16 described a severe case of MP-CAP in a previously healthy woman who presented with acute respiratory distress syndrome (ARDS). These cases underscored early on the potential for fatal outcomes in patients with MP-CAP, even in the absence of significant documented comorbidities. Similar to the cases presented above, these patients presented with normal white blood cell counts, and only one required mechanical ventilation, meeting criteria for severe CAP.
Our cases are particularly noteworthy because their initial chest X-rays were unremarkable and reported as “normal”, which is atypical for MP-CAP.17 MP-CAP is often called “walking pneumonia” because it is known to cause more prominent radiographic findings than clinical signs and symptoms. However, it’s crucial to consider that radiographic findings should be interpreted in conjunction with clinical and laboratory data and that a CT of the chest may be necessary to diagnose CAP in certain scenarios if the initial chest X ray is normal and the patient presents signs and symptoms of pneumonia with hypoxemia.18
Laboratory findings can be helpful in cases of suspected MP-CAP. A 2009 study in Asia involving 1,193 hospitalized patients with CAP found that MP was confirmed in 78 individuals (6%).19 Of those with MP-CAP, 3.7% required non-invasive ventilation, and 4.5% were admitted to the ICU. In this study, the CURB-65 score was between 2-5 in 42.5% of patients, contrasting with our patients for whom the score was much less helpful. The same study identified several laboratory values and factors independently associated with an “atypical pathogen” infection compared to typical bacterial pneumonia. These included: age under 65 years, female gender, fever ≥38.0°C, respiratory rate <25 breaths per minute, pulse rate <100 beats per minute, absence of hyponatremia (serum sodium >130 mmol/L), leukocyte count <11 x 10^9/L, and hemoglobin <11 g/dL. It’s important to note that the term “atypical pathogen” in this study referred to Mycoplasma or Chlamydophila pneumoniae and co-infection with bacteria/viruses, and the results did not specifically differentiate between viral and “atypical” bacterial infections.
While certain risk factors have been linked to an increased risk of CAP in general, their role in increasing MP-CAP severity remains less clear. Both of our patients presented with unique risk factors worth examining.
Case 1 Risk Factors for Severe MP-CAP
The first patient had multiple comorbidities, primarily related to heart disease, and a history of smoking and vaping. It is essential to analyze these factors individually to determine their contribution to the severity of his pneumonia.
Cardiac Disease and Pneumonia Risk
A 2011 retrospective observational study in Asia by Yeh et al.20 evaluated the relationship between acquired cardiovascular disease (CVD) and pneumonia using two cohorts: one with CVD (n = 28,363) and one without CVD. This study demonstrated a significant association between CVD and pneumonia (p<0.001), finding that patients with CVD had an increased risk of pneumonia regardless of age, gender, comorbidity, and antibiotic use. A 2017 systematic review by Almirall et al.21 concluded that numerous risk factors can influence outcomes in patients with pneumonia. While this review established a correlation between smoking and pneumonia, it did not demonstrate a clear association between chronic heart disease and pneumonia risk.
Patients with congenital heart diseases, like aortic coarctation, often exhibit altered immune responses, including abnormalities in immune cell counts and maturation.22 This impaired immune function can contribute to increased morbidity from common pathogens, including MP, usually associated with mild CAP and managed as outpatient. In our first case, the combination of aortic coarctation, aortic stenosis, and a history of smoking and vaping likely contributed to create a scenario that predisposed him to more severe MP-CAP.
While the exact mechanism linking CVD and severe pneumonia is not fully understood and requires further study, several factors may contribute. Aortic coarctation and aortic stenosis can impair blood flow and reduce perfusion to various organs, including the lungs, potentially hindering their function due to possible impaired immune cell recruitment. Both conditions also demand compensatory mechanisms, leading to ventricular hypertrophy and increased afterload, further compromising perfusion efficiency.23 This altered blood flow and increased cardiac strain can lead to reduced oxygenation and inefficient pathogen clearance. The above factors may impact the immunological response early on when the innate immune system arrives to the active site of infection.
Smoking and Vaping as Risk Factors for Severe MP-CAP
Smoking and vaping are well-established risk factors for CAP in general, and their role in increasing disease severity is supported by extensive research.24–26 Several studies highlight the correlation between smoking and increased CAP severity. A 2019 systematic review and meta-analysis by Baskaran et al.27 including 27 studies with a total of 460,592 participants showed that individuals who had ever smoked or were current smokers had a higher risk of developing pneumonia compared to those who had never smoked.
A recent retrospective study in the Netherlands (2023-2024)10 found that 17 patients were admitted to ICUs due to MP-CAP, with five requiring invasive mechanical ventilation. The median age of these patients was 44 years, with a male predominance (82%). Over half (53%) had underlying comorbidities, and 40% had a history of smoking. A study conducted in France28 described 11 patients with MP-CAP, two of whom required ICU admission. The average age was 45.5 years, with 72.7% male predominance. Although they did not have major comorbidities, almost half (45.5%) were smokers. Both studies highlight not only the presence of risk factors like smoking but also suggest an increase in hospitalizations due to MP-CAP in recent years following the COVID-19 pandemic.
The mechanisms by which smoking causes lung damage are well-understood. Cigarette smoke contains numerous reactive molecules that contribute to oxidative stress in membrane lipids, proteins, and cellular organelles.25 This oxidative stress damages the respiratory epithelium, compromising the primary defense mechanism against various agents, including microorganisms, and impairs mucociliary clearance. While more research is needed, vaping has also been shown to have similar adverse effects as smoking, negatively impacting pulmonary function.26 Consequently, the lungs become more susceptible to colonization and invasion by pathogens, including MP. Chronic exposure to irritants from smoking or vaping can trigger persistent lung inflammation, exacerbating infection severity and potentially leading to a more severe clinical course.
Beyond direct damage to the respiratory epithelium, both vaping and smoking can also disrupt the local immune response within the lungs.24–26 Cigarette smoke has been shown to suppress the immune system, affecting immune cell survival, activation, and differentiation. While it increases the number of alveolar macrophages, it impairs their ability to phagocytose bacteria, compromising the clearance of infections.25 This observation may help explain why smokers and vapers are at an increased risk of developing more severe infections, including those caused by common pathogens like MP.
In summary, the combination of structural heart defects (aortic coarctation and aortic stenosis) and respiratory damage from smoking or vaping can synergistically compromise lung structure and function. These conditions likely increased the risk of developing complications from MP infection in our first patient that ended up with hypoxemia and hospitalization.
Case 2 Risk Factors for Severe MP-CAP
The second patient had morbid obesity (BMI 47). While overweight and heart disease can alter immune responses and contribute to disease development, their direct role as risk factors for MP-CAP remains unclear.21 However, obesity has emerged as a significant negative prognostic factor in respiratory infections, as evidenced during the H1N1 and COVID-19 pandemics.29,30
Obesity and Respiratory Infection Severity
Several mechanisms may explain the association between obesity and worse clinical outcomes in patients with MP-CAP. Individuals with obesity often have altered thoracic-abdominal anatomy, leading to impaired diaphragmatic function and reduced lung volumes. These structural changes impede the normal mechanics of ventilation. Excess abdominal fat can displace the diaphragm upward, restricting its movement, while chest wall adiposity can compress the thoracic cage, resulting in shallow breathing and poor ventilation, particularly in the lower lung fields.31 This can impair clearance of respiratory secretions and create an environment conducive to infection.
Obesity is also linked to chronic inflammation, with adipose tissue producing pro-inflammatory cytokines like TNF-alpha, IL-6, and CRP.32 A recent study in mice infected with Pseudomonas aeruginosa demonstrated upregulation of prostaglandin E2 (PGE2) and cyclooxygenase-2 (COX-2) in obese mice, which can impair macrophage phagocytosis and bacterial clearance.33 A review article by Peter Mancuso32 discussed several studies examining the relationship between obesity and the risk of CAP. While a definitive causal link has not been established and further research is needed, the systemic inflammation associated with obesity suggests that obese individuals may be at increased risk of developing severe respiratory infections. This persistent inflammatory state can dysregulate immune responses and promote immune-senescence, making them less effective at combating infections, including those caused by MP.
Obesity is frequently accompanied by comorbidities such as type 2 diabetes, hypertension, and cardiovascular disease, all of which can compromise the body’s ability to fight infections. Diabetes is known to impair neutrophil function and phagocytosis, which are critical for controlling bacterial infections. This impairment reflects a more global immune dysfunction rather than isolated neutrophil dysfunction, leading to a less effective immune response.34,35 Diabetes is also linked to insulin resistance and metabolic syndrome36,37 further contributing to immune dysfunction and increased infection susceptibility. Many individuals with morbid obesity also experience obstructive sleep apnea. The intermittent hypoxemia and production of free radicals associated with sleep apnea can exacerbate the pro-inflammatory state inherent in obesity,38 leading to immune dysfunction, oxidative stress, and increased vulnerability to infection. The combination of impaired immune responses and reduced oxygen delivery to vital tissues further increases the risk of developing severe pneumonia. In obese patients, the severity of pneumonia symptoms, such as shortness of breath, can be significantly amplified due to the additional respiratory compromise caused by obesity. This respiratory distress can contribute to a more severe clinical presentation and may require more aggressive treatment interventions.
In conclusion, morbid obesity may predispose individuals to more severe MP-CAP through a combination of factors, including: impaired respiratory mechanics, altered immune responses, increased comorbidity burden, systemic inflammation and immune-senescence, and compromised pulmonary defense mechanisms. These factors collectively increase the risk of severe infection and complications, making what is typically a mild infection a potentially life-threatening disease.
Diagnostic Challenges in MP-CAP
Diagnosing MP infection can be challenging, and failure to consider this pathogen can lead to misdiagnosis, delayed treatment, and inappropriate antibiotic selection. Sputum is the preferred specimen for PCR testing for MP in adult patients with CAP.39 According to the CDC, nucleic acid amplification tests (NAATs), including PCR, are the preferred diagnostic method for MP infections.40 While PCR is a valuable diagnostic tool, antibody testing, which requires 2 blood samples weeks apart, is often not performed and is not very helpful in clinical practice. On case 2 however, the IGM was (+) and the IGG was (-) which is what it is supposed to occur during an acute infection. Serologies for case 1 were not available.
As reported in the literature median procalcitonin levels are low positive or negative on patients with MP-CAP, as in our two cases.
“….Clinicians may need to be educated about the possibility of facing a case of severe CAP due to MP with negative initial procalcitonin and a chest X ray that can be reported as “normal” which can be highly misleading holding empiric antibiotics…”
A 2021 case report by Metz et al.41 illustrates the consequences of misdiagnosis. A 36-year-old patient was initially diagnosed with COVID-19 based on his presentation with hypoxemia, diffuse interstitial pattern on chest imaging, and the ongoing pandemic. The patient was transferred to the ICU and intubated, and received meropenem without atypical coverage. A bronchoalveolar lavage (BAL) obtained on admission later tested positive for MP. The patient also developed hemolysis, leading to a diagnosis of ARDS with cold agglutinin disease. Similarly, a case report by Sztrymf42 described a 35-year-old woman with Trisomy 21 who was admitted to the ICU with ARDS, also facing delays in MP diagnosis. These cases underscore the importance of considering MP as a potential cause of severe pneumonia and associated complications, especially in patients presenting with relevant risk factors.
Empiric Treatment for MP-CAP
The 2019 ATS/IDSA guidelines for CAP recommend either a beta-lactam/fluoroquinolone combination or a beta-lactam/macrolide combination for severe CAP, with stronger evidence supporting the latter.43 However, the emergence of macrolide-resistant MP may require alternative treatments, such as tetracyclines (doxycycline) or fluoroquinolones as initial empiric regimens.44 In 2018, the average macrolide resistance rate for MP in the US was 7.5%, with a range from 1.9% to 21.7%.45 A 2022 systematic review and meta-analysis by Wang et al.,44 which included 98 investigations spanning 2000-2020 from five continents, examined the prevalence of macrolide-resistant MP. This study revealed geographic variations in resistance rates, with higher rates in Asia. In North America, the overall rate was 8.5%, while individual country rates varied considerably: Korea (2.1%), Japan (25.5%), and China (26.9-100%).
“…Many patients with CAP are treated with beta-lactams only in the outpatient setting without atypical coverage which is recommended by the IDSA/ATS guidelines….”
Mortality Rates for MP-CAP
Recent studies have revealed an increase in hospitalizations attributed to MP infections, highlighting that the mortality rate associated with MP-CAP is often underestimated. A retrospective study by Khoury et al. in Israel46 reviewed 416 patients with MP infection, including 243 adults. The ICU admission rate was 18% for those aged 19-65 years and 38.8% for those over 65 years. The average CURB-65 score for MP patients admitted to the ICU was 1.8 (range 0-5). Mortality was notably high (46.4%) in patients over 65 years. Another study from Japan47 involving 90 patients with MP pneumonia reported that five patients required mechanical ventilation, and three of them died. These findings emphasize the need for increased awareness of MP infections, particularly in severe cases, and underscore the importance of comprehensive diagnostic approaches, appropriate antibiotic selection, and ongoing surveillance of hospitalization trends and outcomes.
Conclusion
The cases presented emphasize that Mycoplasma pneumoniae-related community-acquired pneumonia (MP-CAP), though often considered a mild “walking pneumonia,” can lead to severe outcomes requiring hospitalization, particularly in patients with underlying risk factors like cardiovascular disease, smoking, and morbid obesity. Recognition of MP-CAP as a potential cause of severe pneumonia is crucial, especially in cases where chest radiographs appear normal or initial biomarkers such as procalcitonin are low.
This article contributes significantly to the clinical understanding of MP-CAP by underscoring the importance of comprehensive diagnostic approaches, including the use of chest CT scans when initial imaging is inconclusive, and molecular diagnostic tools for timely identification. Early recognition and appropriate antibiotic selection, particularly in the context of rising macrolide resistance, are key to improving patient outcomes. By identifying critical risk factors for severe disease, this study enhances the ability of clinicians to stratify risk and intervene promptly, ultimately optimizing management strategies and reducing morbidity and mortality associated with MP-CAP.